Better Visibility & Intelligence for Pharma Cold Chains
Deliver vaccines, drugs, and other temperature-sensitive pharma products on time and in full (OTIF), meet strict quality controls (QC), and adhere consistently to pharma safety standards with active monitoring and early warning to mitigate business disruptions across land, air, and ocean modes.
Challenges with Pharma Cold Chain Visibility
In the pharma cold chain, passive or active data loggers are the go-to tracking method, a choice that entails specific challenges.
CHALLENGE #1
Manual touchpoints in passive data logging making data capture delayed and unusable for preventive action.
CHALLENGE #2
Visibility is limited to basic location and condition, even if the data captured is in real-time.
CHALLENGE #3
Inability to identify the root cause of excursions nor foresee the impact on quality control (QC) or stock planning.
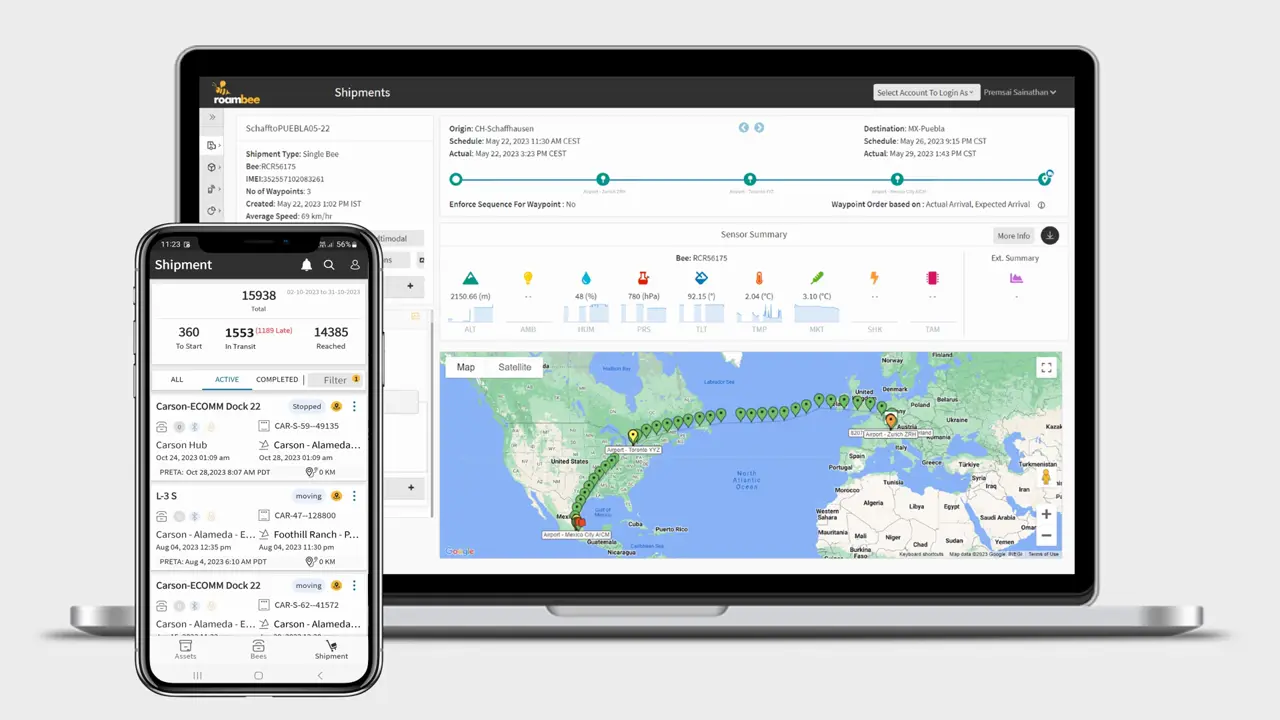
How Roambee's Solution Works for Pharma Cold Chains
Visibility
Better visibility by combining order information with real-time location and condition data from NIST-certified IoT sensors and data streams from port/airport operations, vessel/airline traffic, freight forwarders, and carrier data aggregation platforms.
Alerts & Signals
Built on verifiable visibility, alerts, & signals on a 21 CFR Part 11 compliant platform for better decisions and automation. For example, a business signal about quality compliance (QC) is derived by sensing temperature and handling to mitigate supply risk.
Intelligence
Use AI-powered network insights & foresights for better supply chain planning & execution. For example, score quality compliance (QC) performance by lane, carrier, port, and airport and forecast this key performance indicator (KPI).
Experience the Power of Roambee
Experience the Power of Roambee
Alerts & Business Signals to Help You Make the Right Decisions
Empower your decision-making with real-time alerts and actionable business insights, ensuring timely responses to any disruptions or anomalies in your pharma cold chain operations.
Shipment Signals

Loading & Unloading

Departure & Arrivals

Route Deviation

Customs Clearance

Electronic Proof of
Delivery (ePoD)

Temperature & Humidity
Excursion

Mean Kinetic
Temperature (MKT)

Time Out of
Refrigeration (TOR)

Tilt & Shock

ETA & Delay Signals

Pick-up & Delivery

Location & Geofencing

Authorized & Unauthorized
Container Door Opening

Authorized & Unauthorized
Package Door Opening

Storage

Security Screening

Loading & Unloading

Departure & Arrivals

Route Deviation

Customs Clearance

Electronic Proof of
Delivery (ePoD)

Temperature & Humidity
Excursion

Mean Kinetic
Temperature (MKT)

Time Out of
Refrigeration (TOR)

Tilt & Shock

ETA & Delay Signals

Pick-up & Delivery

Location & Geofencing

Authorized & Unauthorized
Container Door Opening

Authorized & Unauthorized
Package Door Opening

Storage

Security Screening
Business Signals
Delivery Time Windows

Order Fulfilment

Revenue Recognition

Delivery Time Windows

Order Fulfilment

Revenue Recognition

Cold Chain Compliance Prediction

Chain of Custody Breaches

Cargo Handling (Damage)

Cold Chain Compliance Prediction

Chain of Custody Breaches

Cargo Handling (Damage)

Airport Operations Anomalies: Consolidation, Wrong/Missed Connections, Customs, etc.

Port Operations Anomalies: Wrong/Missed Connections, Customs Processing, etc.

Flight Connections Through Unauthorized Countries

Airport Operations Anomalies: Consolidation, Wrong/Missed Connections, Customs, etc.

Port Operations Anomalies: Wrong/Missed Connections, Customs Processing, etc.

Flight Connections Through Unauthorized Countries

Shipment Security Risk

Shipment Security Risk
Business Outcomes with
Roambee
Business Outcomes with Roambee
Revolutionize your pharma cold chain operations with Roambee. Ensure global delivery fulfillment, high cold chain compliance, and flawless order execution. Boost revenue, maintain quality stock, and reduce wastage for unparalleled efficiency.
- Automate the quality control (QC) and proof of delivery (PoD) processes remotely for delivery fulfillment worldwide.
- Mitigate business risks from quality or security compromises in the chain of custody to build a high cold chain compliance score.
- Increase revenue by delivering a flawless order fulfillment experience and transparency.
- Increase revenue by maintaining quality stock at every distribution center to serve patients on time.
- Reduce inventory holding costs with predictable deliveries and increase sustainability with lesser wastage owing to rejected goods.
Empowering you with our Intelligence
Unlock the potential of AI-powered insights for pharma cold chains. Delve into performance metrics, understand operations, and predict challenges. With Roambee, navigate risks, refine your supply chain, and consistently achieve QC and OTIF targets.

Insights
- OTIF performance scoring by lanes, carriers, and nodes.
- Quality compliance (QC) performance across lanes, carriers, and nodes for cold chain and handling.
- Dwell-time/Turnaround-time (TAT) insights into airports, ports, and transshipment operations.
Foresights
- Supply risk prediction at any given node on any given day.
- OTIF and cold chain compliance SLA (service level agreements) predictions by lanes and carriers.
- Handling and security risk predictions at airports, ports, transshipment locations, and during the first/last mile.
Automating Quality Control for a Global Pharma Powerhouse
- About: A leading US-based pharmaceutical giant sought to automate its quality control processes for in-transit vaccine shipments.
- Roambee's Intervention: Using Roambee's business signals, the company could predict the quality acceptance of shipments days before arrival. Roambee identified the top 20 lanes out of 500+ that had six times the average stoppage times. Additionally, quality compliance was showcased by airline, carrier, and transporter on these lanes to strategize temperature and security risk mitigations.
- Results: The interventions led to a significant reduction in stoppage times from an average of 24 days to 4-5 days.
- Impact: The company achieved cost savings, reduced supply risks, and enhanced sustainability by minimizing wastage.
Resources on Visibility & Intelligence

Webinar
Part 1: Telematics Aggregation vs. Sensor Driven Supply Chain Visibility

E-book
Making Smart Supply Chain Decision in the New Normal



